Nitric Oxide Properties Chemistry . Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. The three properties that most determine this chemistry are that •no is small,. Nitric oxide is a colourless paramagnetic gas.
from www.linstitute.net
Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. The three properties that most determine this chemistry are that •no is small,. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). Nitric oxide is a colourless paramagnetic gas. It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction.
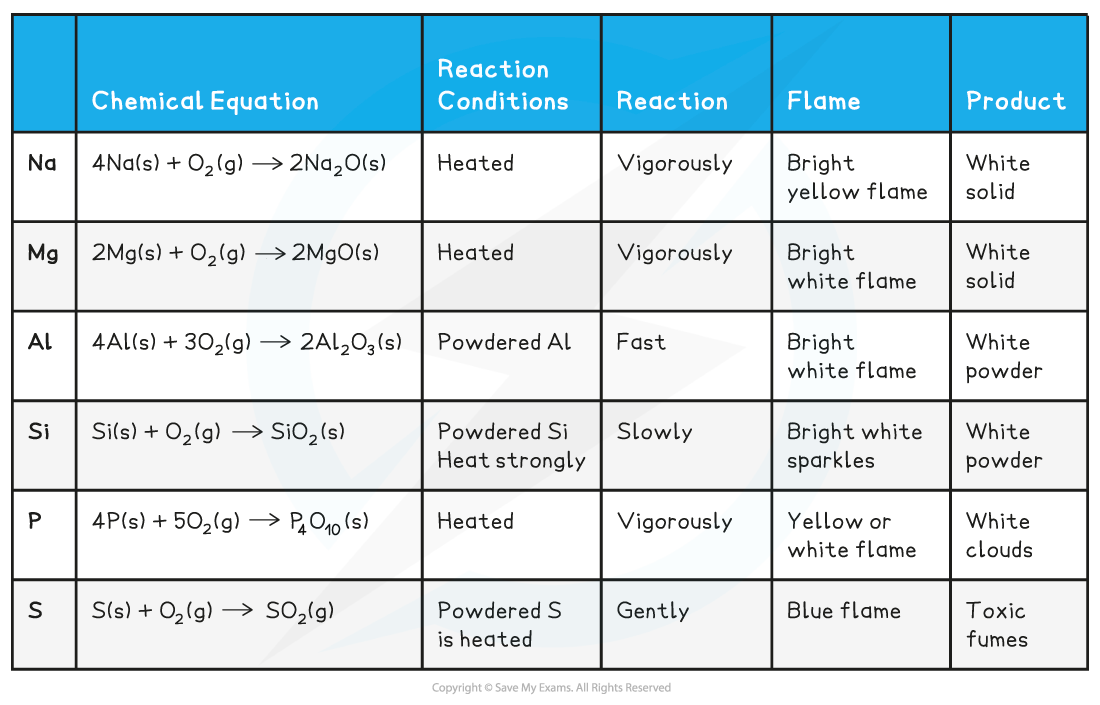
CIE A Level Chemistry复习笔记2.1.3 Period 3 Oxides翰林国际教育
Nitric Oxide Properties Chemistry The three properties that most determine this chemistry are that •no is small,. Nitric oxide is a colourless paramagnetic gas. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. The three properties that most determine this chemistry are that •no is small,. It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ).
From mindthegraph.com
Nitric oxide molecule Nitric Oxide Properties Chemistry Nitric oxide is a colourless paramagnetic gas. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). Nitric oxide is represented by its chemical formula no, is a fascinating. Nitric Oxide Properties Chemistry.
From www.slideserve.com
PPT Nitric Oxide PowerPoint Presentation, free download ID183938 Nitric Oxide Properties Chemistry The three properties that most determine this chemistry are that •no is small,. It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide is a colourless paramagnetic gas. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide. Nitric Oxide Properties Chemistry.
From present5.com
Biological Effects of Nitric Oxide and its Role Nitric Oxide Properties Chemistry It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). The three properties that. Nitric Oxide Properties Chemistry.
From material-properties.org
Nitrous Oxide Density, Heat Capacity, Thermal Conductivity Nitric Oxide Properties Chemistry Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. The three properties that most determine this chemistry are that •no is small,. Nitric oxide. Nitric Oxide Properties Chemistry.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.1.4 Period 3 Oxides & Hydroxides Acid/Base Nitric Oxide Properties Chemistry Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. The three properties that most determine this chemistry are that •no is small,. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent. Nitric Oxide Properties Chemistry.
From franklincardiovascular.com
NITRIC OXIDE IN PROMOTING HEALTHY AUTONOMIC FUNCTION Autonomic Nitric Oxide Properties Chemistry Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. Nitric oxide is a colourless paramagnetic gas. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). It is made, for example, by the reduction of concentrated nitric acid. Nitric Oxide Properties Chemistry.
From www.dreamstime.com
Nitric Oxide NO Free Radical and Signaling Molecule. 3D Rendering Nitric Oxide Properties Chemistry The three properties that most determine this chemistry are that •no is small,. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). Nitric oxide is a colourless paramagnetic gas.. Nitric Oxide Properties Chemistry.
From www.spnathletes.com
The Benefits of Nitric Oxide Nitric Oxide Properties Chemistry Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide is a colourless paramagnetic gas. It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and. Nitric Oxide Properties Chemistry.
From www.youtube.com
Oxides across period 3 IB Chemistry Revision Course YouTube Nitric Oxide Properties Chemistry It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the. Nitric Oxide Properties Chemistry.
From pediaa.com
Difference Between Nitric Oxide and Nitrous Oxide Definition Nitric Oxide Properties Chemistry Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. The three properties that most determine this chemistry are that •no is small,. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide. Nitric Oxide Properties Chemistry.
From www.researchgate.net
1 The various routes of nitric oxide formation in plants. Reductive Nitric Oxide Properties Chemistry The three properties that most determine this chemistry are that •no is small,. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide is a colourless paramagnetic gas. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ).. Nitric Oxide Properties Chemistry.
From www.youtube.com
Oxides, its classification and different properties of oxides YouTube Nitric Oxide Properties Chemistry Nitric oxide is a colourless paramagnetic gas. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). The three properties that most determine this chemistry are that •no is small,.. Nitric Oxide Properties Chemistry.
From www.chegg.com
Solved Consider the reaction between nitric oxide and ozone. Nitric Oxide Properties Chemistry Nitric oxide is a colourless paramagnetic gas. The three properties that most determine this chemistry are that •no is small,. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric. Nitric Oxide Properties Chemistry.
From www.youtube.com
N2O liquid Nitrous oxide (Dinitrogen monoxide) chemical reactions Nitric Oxide Properties Chemistry Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. The three properties that. Nitric Oxide Properties Chemistry.
From www.semanticscholar.org
[PDF] Nitric Acid, Nitrous Acid, and Nitrogen Oxides Semantic Scholar Nitric Oxide Properties Chemistry Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide (figure \(\pageindex{6}\)b). Nitric Oxide Properties Chemistry.
From www.slideserve.com
PPT Chemical Equations and Reactions PowerPoint Presentation, free Nitric Oxide Properties Chemistry Nitric oxide is a colourless paramagnetic gas. The three properties that most determine this chemistry are that •no is small,. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron ). It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide (figure \(\pageindex{6}\)b) is electronically. Nitric Oxide Properties Chemistry.
From in.dental-tribune.com
Nitric Oxide the Nobel prize winning molecule, can play a critical Nitric Oxide Properties Chemistry Nitric oxide is a colourless paramagnetic gas. The three properties that most determine this chemistry are that •no is small,. It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide is represented by its chemical formula no, is a fascinating molecular compound that plays a crucial role in the world. Nitric oxide. Nitric Oxide Properties Chemistry.
From classnotes.org.in
Oxides of Nitrogen Chemistry, Class 12, The pBlock Elements Nitric Oxide Properties Chemistry It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. The three properties that most determine this chemistry are that •no is small,. Nitric oxide is a relatively unstable, diatomic molecule. Nitric Oxide Properties Chemistry.